Delta G And K Relationship
19.7: ΔG° and One thousand as Functions of Temperature
- Page ID
- 24320
Learning Objectives
- To know the relationship between free energy and the equilibrium constant.
As was previously demonstrated, the spontaneity of a procedure may depend upon the temperature of the system. Phase transitions, for example, will proceed spontaneously in one direction or the other depending upon the temperature of the substance in question. Also, some chemical reactions tin can likewise exhibit temperature dependent spontaneities. To illustrate this concept, the equation relating free energy change to the enthalpy and entropy changes for the process is considered:
\[ ΔG=ΔH−TΔS \]
The spontaneity of a process, as reflected in the arithmetic sign of its free energy change, is then determined by the signs of the enthalpy and entropy changes and, in some cases, the accented temperature. Since T is the accented (kelvin) temperature, information technology can only have positive values. Iv possibilities therefore be with regard to the signs of the enthalpy and entropy changes:
- Both ΔH and ΔS are positive. This status describes an endothermic process that involves an increase in system entropy. In this case, ΔG will exist negative if the magnitude of the TΔDue south term is greater than ΔH. If the TΔSouthward term is less than ΔH, the free energy change will be positive. Such a process is spontaneous at high temperatures and nonspontaneous at low temperatures.
- Both ΔH and ΔS are negative. This status describes an exothermic process that involves a decrease in organisation entropy. In this case, Δ1000 volition exist negative if the magnitude of the TΔSouthward term is less than ΔH. If the TΔDue south term's magnitude is greater than ΔH, the free energy change volition be positive. Such a procedure is spontaneous at low temperatures and nonspontaneous at high temperatures.
- ΔH is positive and ΔS is negative. This condition describes an endothermic process that involves a decrease in system entropy. In this case, ΔG will exist positive regardless of the temperature. Such a procedure is nonspontaneous at all temperatures.
- ΔH is negative and ΔS is positive. This condition describes an exothermic process that involves an increase in organisation entropy. In this case, ΔThousand will be negative regardless of the temperature. Such a process is spontaneous at all temperatures.
These 4 scenarios are summarized in Figure \(\PageIndex{ane}\).
Predicting the Temperature Dependence of Spontaneity
The incomplete combustion of carbon is described by the post-obit equation:
\[\ce{2C}(due south)+\ce{O2}(one thousand)⟶\ce{2CO}(g) \nonumber\]
How does the spontaneity of this process depend upon temperature?
Solution
Combustion processes are exothermic (ΔH < 0). This particular reaction involves an increment in entropy due to the accompanying increase in the corporeality of gaseous species (net gain of one mole of gas, ΔS > 0). The reaction is therefore spontaneous (ΔThousand < 0) at all temperatures.
Practice \(\PageIndex{iii}\)
Popular chemical paw warmers generate heat by the air-oxidation of iron:
\[\ce{4Fe}(southward)+\ce{3O2}(g)⟶\ce{2Fe2O3}(southward) \nonumber\]
How does the spontaneity of this process depend upon temperature?
Answer
ΔH and ΔS are negative; the reaction is spontaneous at low temperatures.
When considering the conclusions drawn regarding the temperature dependence of spontaneity, it is important to go on in listen what the terms "high" and "low" mean. Since these terms are adjectives, the temperatures in question are deemed loftier or low relative to some reference temperature. A process that is nonspontaneous at one temperature but spontaneous at another will necessarily undergo a alter in "spontaneity" (as reflected by its ΔGrand) every bit temperature varies. This is clearly illustrated by a graphical presentation of the free energy alter equation, in which ΔG is plotted on the y centrality versus T on the x centrality:
\[ΔG=ΔH−TΔS\]
\[y=b+mx\]
Such a plot is shown in Figure \(\PageIndex{2}\). A process whose enthalpy and entropy changes are of the same arithmetic sign will exhibit a temperature-dependent spontaneity equally depicted by the ii yellow lines in the plot. Each line crosses from one spontaneity domain (positive or negative ΔM) to the other at a temperature that is characteristic of the process in question. This temperature is represented by the x-intercept of the line, that is, the value of T for which ΔK is zero:
\[ΔG=0=ΔH−TΔS\]
\[T=\dfrac{ΔH}{ΔS}\]
And then, saying a process is spontaneous at "high" or "low" temperatures means the temperature is to a higher place or below, respectively, that temperature at which ΔG for the process is zero. As noted earlier, this status describes a organization at equilibrium.
Equilibrium Temperature for a Stage Transition
As defined in the chapter on liquids and solids, the boiling signal of a liquid is the temperature at which its solid and liquid phases are in equilibrium (that is, when vaporization and condensation occur at equal rates). Apply the information in Appendix G to estimate the boiling point of water.
Solution
The procedure of interest is the following phase alter:
\[\ce{H2o}(50)⟶\ce{H2O}(g) \nonumber\]
When this process is at equilibrium, ΔOne thousand = 0, so the following is truthful:
\[0=ΔH°−TΔS°\hspace{40px}\ce{or}\hspace{40px}T=\dfrac{ΔH°}{ΔS°} \nonumber\]
Using the standard thermodynamic information from Appendix G,
\[\brainstorm{marshal}
ΔH°&=ΔH^\circ_\ce{f}(\ce{Water}(one thousand))−ΔH^\circ_\ce{f}(\ce{Water}(l)) \nonumber\\
&=\mathrm{−241.82\: kJ/mol−(−285.83\: kJ/mol)=44.01\: kJ/mol} \nonumber
\end{align}\]
\[\begin{marshal}
ΔS°&=ΔS^\circ_{298}(\ce{H2O}(g))−ΔS^\circ_{298}(\ce{Water}(l)) \nonumber\\
&=\mathrm{188.8\: J/G⋅mol−70.0\: J/G⋅mol=118.viii\: J/Thou⋅mol} \nonumber
\terminate{align}\]
\[T=\dfrac{ΔH°}{ΔS°}=\mathrm{\dfrac{44.01×10^3\:J/mol}{118.8\:J/Thou⋅mol}=370.5\:K=97.iii\:°C} \nonumber\]
The accustomed value for water'due south normal boiling point is 373.2 K (100.0 °C), and so this calculation is in reasonable agreement. Annotation that the values for enthalpy and entropy changes data used were derived from standard information at 298 K (Appendix G). If desired, y'all could obtain more authentic results past using enthalpy and entropy changes determined at (or at to the lowest degree closer to) the actual boiling point.
Practise \(\PageIndex{four}\)
Use the information in Appendix G to estimate the boiling betoken of CS2.
Answer
313 K (accepted value 319 One thousand)
Temperature Dependence of the Equilibrium Constant
The fact that ΔG° and K are related provides us with another caption of why equilibrium constants are temperature dependent. This human relationship can be expressed as follows:
\[\ln K=-\dfrac{\Delta H^\circ}{RT}+\dfrac{\Delta S^\circ}{R} \label{18.xl}\]
Assuming ΔH° and ΔS° are temperature independent, for an exothermic reaction (ΔH° < 0), the magnitude of K decreases with increasing temperature, whereas for an endothermic reaction (ΔH° > 0), the magnitude of Yard increases with increasing temperature. The quantitative relationship expressed in Equation \(\ref{18.twoscore}\) agrees with the qualitative predictions made past applying Le Chatelier'south principle. Because oestrus is produced in an exothermic reaction, adding heat (by increasing the temperature) will shift the equilibrium to the left, favoring the reactants and decreasing the magnitude of M. Conversely, because heat is consumed in an endothermic reaction, adding heat will shift the equilibrium to the right, favoring the products and increasing the magnitude of Thou. Equation \(\ref{18.40}\) also shows that the magnitude of ΔH° dictates how rapidly K changes equally a function of temperature. In contrast, the magnitude and sign of ΔS° bear upon the magnitude of Chiliad but not its temperature dependence.
If we know the value of K at a given temperature and the value of ΔH° for a reaction, we tin estimate the value of K at any other temperature, even in the absenteeism of data on ΔS°. Suppose, for example, that K1 and Chiliad2 are the equilibrium constants for a reaction at temperatures T1 and T2, respectively. Applying Equation \(\ref{18.40}\) gives the following relationship at each temperature:
\[\begin{marshal}\ln K_1&=\dfrac{-\Delta H^\circ}{RT_1}+\dfrac{\Delta South^\circ}{R}
\\ \ln K_2 &=\dfrac{-\Delta H^\circ}{RT_2}+\dfrac{\Delta S^\circ}{R}\end{align}\]
Subtracting \(\ln K_1\) from \(\ln K_2\),
\[\ln K_2-\ln K_1=\ln\dfrac{K_2}{K_1}=\dfrac{\Delta H^\circ}{R}\left(\dfrac{i}{T_1}-\dfrac{1}{T_2}\right) \label{eighteen.41}\]
Thus computing ΔH° from tabulated enthalpies of germination and measuring the equilibrium constant at i temperature (K1) allow u.s. to calculate the value of the equilibrium constant at any other temperature (K2), bold that ΔH° and ΔS° are independent of temperature. The linear relation between \(\ln Chiliad \)and the standard enthalpies and entropies in Equation \(\ref{18.41}\) is known as the van't Hoff equation. It shows that a plot of \(\ln G\) vs. \(1/T\) should be a line with slope \(-\Delta_r{H^o}/R\) and intercept \(\Delta_r{Southward^o}/R\).
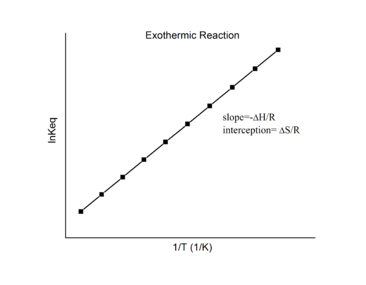
Hence, these thermodynamic enthalpy and entropy changes for a reversible reaction tin can be determined from plotting \(\ln K\) vs. \(i/T\) data without the help of calorimetry. Of course, the main assumption hither is that \(\Delta_r{H^o}\) and \(\Delta_r{S^o}\) are merely very weakly dependent on \(T\), which is usually valid over a narrow temperature range.
Example \(\PageIndex{iv}\)
The equilibrium constant for the formation of NHthree from Hii and N2 at 25°C is Kp = 5.iv × 105. What is Kp at 500°C? (Utilize the information from Case x.)
Given: balanced chemical equation, ΔH°, initial and terminal T, and Kp at 25°C
Asked for: Kp at 500°C
Strategy:
Convert the initial and final temperatures to kelvins. And then substitute appropriate values into Equation \(\ref{18.41}\) to obtain Thou2, the equilibrium constant at the terminal temperature.
Solution:
The value of ΔH° for the reaction obtained using Hess'due south law is −91.viii kJ/mol of Nii. If we gear up T1 = 25°C = 298.K and T2 = 500°C = 773 K, and then from Equation \(\ref{18.41}\) we obtain the post-obit:
\(\begin{align}\ln\dfrac{K_2}{K_1}&=\dfrac{\Delta H^\circ}{R}\left(\dfrac{i}{T_1}-\dfrac{1}{T_2}\right)
\\&=\dfrac{(-\textrm{91.8 kJ})(\textrm{1000 J/kJ})}{\textrm{8.314 J/K}}\left(\dfrac{1}{\textrm{298 K}}-\dfrac{1}{\textrm{773 K}}\right)=-22.8
\\ \dfrac{K_2}{K_1}&=1.3\times10^{-ten}
\\ K_2&=(five.four\times10^5)(1.3\times10^{-x})=vii.0\times10^{-5}\end{align}\)
Thus at 500°C, the equilibrium strongly favors the reactants over the products.
Exercise \(\PageIndex{4}\)
In the exercise in Example \(\PageIndex{3}\), you calculated Yardp = 2.two × 1012 for the reaction of NO with O2 to give NOii at 25°C. Use the ΔH∘ f values in the exercise in Example x to calculate Gp for this reaction at 1000°C.
Answer: 5.6 × 10−4
The Van't Hoff Equation: https://youtu.be/4vk6idAXp_A
Summary
For a reversible process that does not involve external work, we can express the change in free energy in terms of volume, pressure, entropy, and temperature. If nosotros presume ideal gas behavior, the ideal gas police force allows us to limited ΔG in terms of the partial pressures of the reactants and products, which gives us a human relationship between ΔG and Kp, the equilibrium abiding of a reaction involving gases, or K, the equilibrium constant expressed in terms of concentrations. If ΔG° < 0, then Grand or Mp > 1, and products are favored over reactants. If ΔG° > 0, then K or Mp < one, and reactants are favored over products. If ΔG° = 0, then K or 1000p = i, and the arrangement is at equilibrium. Nosotros tin can utilize the measured equilibrium constant K at ane temperature and ΔH° to approximate the equilibrium abiding for a reaction at whatsoever other temperature.
Delta G And K Relationship,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/19%3A_Spontaneous_Change%3A_Entropy_and_Gibbs_Energy/19.7%3A_G_and_K_as_Functions_of_Temperature
Posted by: weaverdecroure.blogspot.com


0 Response to "Delta G And K Relationship"
Post a Comment